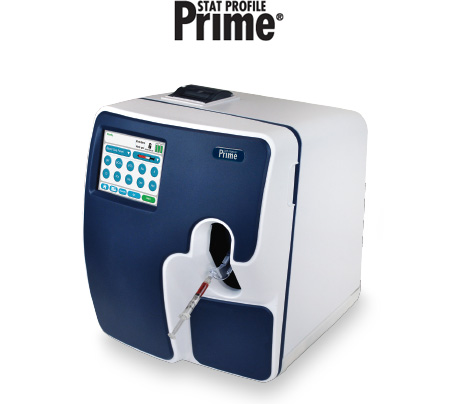
Stat Profile Prime® Analyzer
The New Definition of
Simplicity for Critical Care Testing
Measures: pH PCO2 PO2 Na K Cl iCa Glu Lac Hct
Stat Profile Prime combines the revolutionary microelectronics of the consumer world with Nova Biomedical’s innovative MicroSensor Card™ technology for a smaller, faster, more powerful yet simpler and less expensive analyzer.
Maintenance-free cartridge system
Stat Profile Prime’s unique cartridge system consists of individual cartridges for sensors, calibrators, and liquid quality control (QC).Maximum analyzer uptime
Individual cartridges offer a significant benefit in analyzer uptime compared to combined sensor/calibrator cartridge systems.Cartridge replacement in seconds
Each cartridge is ready to use and easily replaced in seconds. Cartridge RFID technology automatically captures cartridge installation time, date, lot number, test parameters, and usage. There is significant savings in analyzer uptime when replacing only a calibrator cartridge, which has no warm-up time, compared to a two-to-four-hour wait for combined cartridge systems.Individual cartridges lower costs
Individual cartridges offer a low-cost alternative compared to the inflexibility and waste of combined cartridge systems. For example, an analyzer used in a high volume setting will require fewer sensor cartridges than calibrators, but a low volume setting will require the reverse. In both cases, costs are reduced by using Stat Profile Prime’s individual sensor and calibrator cartridges, which have no waste compared to combined cartridge systems.MicroSensor card technology
AccuracyAll Stat Profile Prime tests use proven Nova biosensor technology in a miniaturized, sensor card format.
Constant stat readiness
MicroSensor cards have flexible configurations:
- 200 Samples
- 400 Samples
MicroSensor cards are automatically calibrated and always ready for immediate analysis.
 Clot protection
Clot protection
The unique Clot Block™ sample flow path is designed to protect sensor cartridges from blood clot blockages.
Fastest replacement time
MicroSensor cards can be replaced, warmed, and calibrated in less than half the time of other cartridge systems, which take more than an hour to calibrate and can remain unstable with drift, frequent re-calibrations, and reduced throughput for even longer periods of time.
Automated, true liquid QC
True liquid QC provides the only reliable test of an analyzer In the U.S., federal government regulations (CLIA) are phasing out electronic QC and are requiring true, liquid-based QC. Stat Profile Prime combines both automated, true liquid QC
and continuous electronic self-monitoring to ensure lab accuracy and uncompromised quality.
In the U.S., federal government regulations (CLIA) are phasing out electronic QC and are requiring true, liquid-based QC. Stat Profile Prime combines both automated, true liquid QC
and continuous electronic self-monitoring to ensure lab accuracy and uncompromised quality.
Tri-level QC cartridges automate daily QC
QC cartridges contain a 30-day supply of liquid QC material. Controls are run automatically at user-selected intervals. An optional QC lockout feature disables any analyte that is not within QC limits.
CLIA QC compliant out of the box
Stat Profile Prime’s automated QC system complies with all new CLIA requirements.1 Liquid quality controls follow the identical sample pathway as the blood sample and confirm the entire analytical process for each analyte at multiple levels of concentration. QC statistics are automatically maintained.
Complete automation
Running QC manually is one of the most time consuming aspects of critical care testing. Stat Profile Prime’s automated, true liquid QC saves hours of time each week, eliminating the need to develop, implement, and document a lengthy, risk-based quality assessment and individualized QC plan.
Supplemental quality monitoring
Stat Profile Prime provides a supplement to liquid QC by continuously monitoring the status and performance of all analytical components (including sensors, reagents, calibrations, sample integrity, software, and electronics) providing real-time, sample-to-sample assurance of correct performance.10 critical care tests – results in 60 seconds
- pH PCO2 PO2 Na K Cl iCa Glu Lac Hct
- Fast throughput: Up to 45 samples/hour
- Arterial, venous, or capillary micro-samples: 100 microliters for full menu
Bidirectional connectivity
Stat Profile Prime's bidirectional software uploads patient and QC results to hospital and laboratory information systems using ASTM or POCT1-A2 formats. The bidirectional interface also enables downloading of test orders, patient identifiers, and patient demographics from the LIS or HIS to Stat Profile Prime. Complete, accurate test records are seamlessly captured for the medical record. Also included is comprehensive cybersecurity protection and encryption that protects PHI and provides an added level of protection against for attempts to access a hospital's network.Safe, simple, fast operation
 Syringes can be docked and sampled with
Syringes can be docked and sampled with hands-free operation.
 Hands-free capillary sampling can be performed
Hands-free capillary sampling can be performed without adapters.
 Samples can be aspirated directly from tubes,
Samples can be aspirated directly from tubes, eliminating sample transfer to a syringe or capillary.
 QC proficiency ampules can be sampled without adapters.
QC proficiency ampules can be sampled without adapters.
Easy sampling from syringes, capillaries, tubes, and ampoules
A unique safety sample port protects the user from accidental contact with the sample probe and is easily accessed for all sample containers.
Easy-to-use, high-definition, color touchscreen
The touchscreen is easily operated through the use of simple and intuitive prompts and requires minimal training.
Three simple steps to initiate a full 10-test profile
- Press “Start”
- Scan or enter patient ID
- Press “Aspirate”
Integrated barcode scanner
The 1D/2D barcode scanner, conveniently located within the sample port allows for fast, error-free entry of operator and patient IDs. The optional, wireless, external barcode scanner also allows for positive patient ID, further eliminating pre-analytical error.Compact, point-of-care size
Stat Profile Prime’s microelectronics and cartridge system result in one of the smallest and lightest critical care analyzers. Stat Profile Prime is so compact it can be located virtually anywhere in the hospital or operated on a mobile cart with a battery back-up.

Nova Biomedical / 200 Prospect Street / Waltham, MA 02454 / 781-894-0800
1. Centers for Medicare and Medicaid Services. (2003). Laboratory requirements, quality system for nonwaived testing, subpart K. §493.1256. Retrieved from https://www.ecfr.gov/cgi-bin/text-idx?rgn=div5&node=42:5.0.1.1.9#sp42.5.493.k